electron configuration of rn|rn valence electrons : Tuguegarao Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure. The chemical symbol for Radon is Rn.
Download the Bet88 mobile app to access casino games and sports betting on your mobile device. Experience convenience and fun gaming – get the app now! . We are confident that we can meet the betting needs of our members. Members have more than 4,000 games per month. Online Games. Join online games such as Roulette, blackjack, .
PH0 · rn valence electrons
PH1 · periodic table 1s 2s 2p
PH2 · pb 4+ electron configuration
PH3 · electron periodic table configuration
PH4 · electron configuration worksheet
PH5 · electron configuration of oxygen
PH6 · electron configuration for every element
PH7 · abbreviated electron configuration chart
PH8 · Iba pa
Beauty, beauty and the beast Beauty from the east, beautiful confessions of the priest Beast, beauty from the streets, we don't get deceased Every time a beauty on the beats (Yeah, yeah, yeah, let's go, let's go) Body rock, girl, I wanna feel your body rock Cause all I need Is a beauty and a beat Who can make my life complete It's all about you,
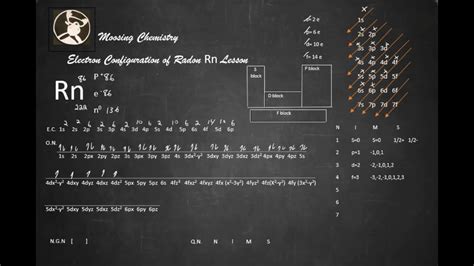
electron configuration of rn*******The arrangement of electrons in radon in specific rules in different orbits and orbitals is called the electron configuration of radon. The electron configuration of radon is [ Xe] 4f 14 5d 10 6s 2 6p 6 , if the electron arrangement is through orbitals. Electron .
Mar 23, 2023 A step-by-step description of how to write the electron configuration for Radon (Rn). In order to write the Rn electron configuration we first need to know the .Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .Radon is a chemical element of the periodic table with chemical symbol Rn and atomic number 86 with an atomic weight of 222 u and is classed as a noble gas.
electron configuration of rn Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure. The chemical symbol for Radon is Rn. Fluorine Electron Configuration. Neon Electron Configuration. The radon is also the immediate decay product of radium. Its most stable 222 Rn, isotope, has a half-life of only 3.8 days which .
Write the electron configuration from your orbital diagram. Ignore the inner orbitals (those that correspond to the electron configuration of the nearest noble gas) and write the .
rn valence electronsWrite the electron configuration from your orbital diagram. Ignore the inner orbitals (those that correspond to the electron configuration of the nearest noble gas) and write the .Comprehensive information for the element Radon - Rn is provided by this page including scores of properties, element names in many languages, most known nuclides and . Radon condensed electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6. It is the same as the ground state .

Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 211 Rn, 220 Rn, 222 Rn Electron configuration [Xe] 4f 1 4 5d 1 0 6s 2 6p 6 CAS number: 10043-92-2 ChemSpider ID .
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .Comprehensive information for the element Radon - Rn is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 f 14 5s 2 p 6 d 10 6s 2 p 6; Electrons per Energy Level: 2,8 .Element 86 of Periodic table is Radon with atomic number 86, atomic weight 222. Radon, symbol Rn, has a Face Centered Cubic structure and Colorless color. Radon is a Noble Gas element. It is part of group 18 (helium family or neon family). Know everything about Radon Facts, Physical Properties, Chemical Properties, Electronic configuration .
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. . radon (Z = 86), [Xe]6s 2 4f 14 5d 10 6p 6 = [Rn]. In the last row, the 5f orbitals are filled between the 7s and the 6d orbitals, which gives the 14 actinide elements. Because the large number of . Rn: properties of free atoms. Radon atoms have 86 electrons and the shell structure is 2.8.18.32.18.8. The ground state electron configuration of ground state gaseous neutral radon is [ Xe ]. 4f14. 5d10. 6s2. 6p6 and the term symbol is 1S0. Schematic electronic configuration of radon. The Kossel shell structure of radon.
Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.
Introducing our new OnePlus Community experience, with a completely revamped structure, built from the ground-up.
electron configuration of rn|rn valence electrons